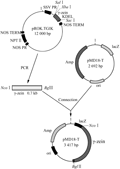
Construction of Plant Expression Vector with Maize γ-zein Gene and GFP Gene and Their Subcellular Localization 
2 Institute of Animal Science, Chinese Academy of Agricultural Science, Beijing, 100094, P.R. China
 Author
Author  Correspondence author
Correspondence author
Maize Genomics and Genetics, 2012, Vol. 3, No. 2 doi: 10.5376/mgg.2012.03.0002
Received: 29 Feb., 2012 Accepted: 30 May, 2012 Published: 01 Jun., 2012
Zhang et al., 2012, Construction of Plant Expression Vector with Maizeγ-zein gene and GFP gene and their Subcellular Localization, Maize Genomics and Genetics, Vol.3, No.2, 6-12 (doi: 10.5376/mgg.2012.03.0002)
The full length of the maize γ-zein gene amplified by PCR from PROK.TG1LK plasmid was ligated into the intermediate vector pMD18-T, to obtain the recombinant plasmid named pMD18-γ-zein. After the recombinant plasmid and the improved pCAMBIAI1302,respectively were digested by Nco I and Bgl II restriction endonucleases, the plant expression vector pCB-GFP-γ-zein with the gene of green fluorescent protein (GFP) driven by 35S was built following the procedures of recovery, ligation, transformation and identification. The fusion of GFP-γ-zein gene was integrated into the onion epidermal cells by using the Biolistic approach; the fusion gene was expressed in onion epidermal cell wall, nucleus and cytoplasm confirmed by confocal microscopy.
In the processes of plant genetic transformation, a reporter gene is necessary to test whether the foreign gene expresses in the receptor cells, tissues or whole plants. As a reporter gene, Green Fluorescent Protein (GFP) is only one of the light-emitting proteins being expressed in living cells.
The green fluorescent protein is a kind of a natural light-emitting protein from the coelenterate Jellyfish (Aequorea vitoria), which composes of 237 amino acids with the molecular weight of approximately 27 kD; GFP excited by ultraviolet light will emit green fluorescence light (Shimomura et al., 1962). Bemdna et al improved the luminous intensity of the GFP to facilitate its detection by using site-directed mutagenesis on the encoding chromophore amino acids and relative amino acids. In addition, the GFP is also converted into being capable of emitting other color fluorescent mutants, for example, the red fluorescent protein, yellow fluorescent protein and blue fluorescent protein.
GFP gene employed as reportor gene is widely applied in plant genetic transformation (Chiu et al., 1996; Elliott et al., 1999; Sumilkumar et al., 2001) due to its advantages of stable fluorescent light emission, convenient detection and non-species specificity, no poisoning to plant as well as being capable of in vivo detection (Huang et al., 1998; Wang et al., 2004); while the GFP has been widely used in positioning, expressing and developing of exogenous genes in the genetically modified plants (Wang et al., 2004; Zhao et al., 2006).
Corn Zein is a storage protein synthesized in the formation of seed, encoded by the multi-gene family. Zein was formed in the rough endoplasmic reticulum and eneven accumulated on the endoplasmic reticulum in the form of protein bodies with the size of 1~2 μmol/L polymer (Jacques, 2002).
Depending on the rate of electrophoretic migration, the zein can be divided into different groups as alpha- zein (the 22 and 19 kD), beta-zein (15 kD), gamma-zein (27 and 16 kD), and δ-zein (18, and 10 kD,) (Esen etal., 1987; Wilson et al., 1991; Coleman, et al., 1998), except for α-zein, β-zein and δ-zein, γ-zein contain sulfur-rich amino acids such as methionine (Met) and cysteine (Cys) (Wooa et al., 2001; Shewry and Casey, 1999), which are two kinds of sulfur- containing amino acids for proteins. Due to their unique structure, methionine (Met) and cysteine (Cys) play important roles in animal nutrition and immune- related physiological functions (Xie real Yong, 2003).
In order to explore the studies of γ-zein gene expression, localization, and related functions in plant cells by taking advantage of the GFP fluorescence characteristics, in this research the full length sequence of the γ-zein gene was amplified by PCR from pROK.TG1LK, which was ligated into plasmid pCAMBIA1302 to make a fusion gene with GFP and then build the plant expression vector named pMD18-γ-zein The fusion of GFP-γ-zein gene would be integrated into the onion epidermal cells by using the Biolistic approach, and expressed in onion epidermal cell wall, nucleus and cytoplasm confirmed by confocal microscopy., lay the foundation for the γ- zein genetic transformation, positioning expression.
1 Result and analysis
1.1 pCAMBIA1302 vector identification
Digestion results showed (Figure 1) that there are two bands digested by Sph I or Xho I, respectively. Two band with a molecular weight of approximately 8.6 kb and 1.9 kb occurs by digestion of single Sph I enzyme (Figure 1A), while two bands with a molecular weight of approximately 9.4 kb and 1.1 kb appears by digestion of single Xho â… (Figure 1B). The sum of their molecular weights (approximately 10.5 kb) is consistent with the fragment of the plasmid pCAMBIA1302.
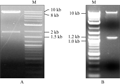 Figure 1 Plant expression vector pCAMBIA1302 identified by restriction enzymetic analysis |
The expected band in size 538 bp of GFP gene was amplified by PCR (Figure 2) that indicates the GFP gene existing in pCAMBIA1302 vector, and the improved plant expression vector pCAMBIA1302 is proven to be integrity by a single restriction enzyme digestion and PCR amplification test, which can be used for further building the expression vector.
.png) Figure 2 PCR identificaton of GFP gene in plant expression vector pCAMBIA1302 |
1.2 Construction of pMD18-γ-zein
The constructing procedure for intermediate expression vector was shown in Figure 3. DNA from Prok.TG1 plasmid as template prepared by Mini-Prep extraction kit; the single band with expected 720 bp in length amplified by PCR using the primer that adds enzymatic restriction sites and protecting nucleotide bases, and get the one with the expected length of 720 bp single band (Figure 4).
|
|
.png) Figure 4 GFP gene amplified by PCR from pROK.TG1LK |
The amplified fragment was recovered and purified prior to adenine-adding reaction, then γ-zein was ligated to pMD18-T overnight under the action of T4 DNA ligase and transformed into competent E. coli DH5α, White colonies being positive clones were picked up to be inoculated in YMB liquid medium (containing 50 μg/mL of Kan, 50 μg/mL, Str), After shaking incubation (28°C, 200 r/min) overnight, the bacteria were identified by PCR (Figure 5), the results of PCR identification were consistent with the expected size that indicated the selected white colonies were positive clones.
The DNA was extracted from the identified positive intermediate vector, and validated with double digestion by restriction enzymes Ncoâ… / Bgl II; the recombinant vector DNA can be cut into about 2.6 kb pMD18-T linear band and about 720 bp γ-the zein bands obtained after double enzymatic digestion (Figure 6). The results showed that the size of the digested bands were consistent with the expected sizes, which indicated the construction of the vector should be correct, we named the positive recombinant plasmid as pMD18-γ-zein containing the γ-zein ligated into the pMD18-T.
| Figure 5 GFP gene PCR identification of intermediate vector pMD18-γ-zein |
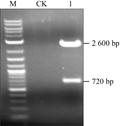 Figure 6 Restriction analysis of intermediate vector pMD18-γ-zein |
1.3 Construction of plant expression vector pCB-GFP-γ-zein
The procedure to build plant expression vector showed in Figure 7. The DNAs of vector pCAMBIA1302 and pMD18-γ zein were extracted respectively, and digested with Ncoâ… /Bgl II restriction enzymes. After electrophoresis, about 10 kb linear fragment of pCAMBIA1302 and about 0.7 kb γ-zein fragment were recovered, respectively.
The recovered 0.7 kb γ-zein fragment ligated into the linear fragment of 10 kb of pCAMBIA1302 under the reaction of T4 DNA Ligase and then transformed into competent E. coli to generate the recombinant, which were validated by PCR (Figure 8) and restriction endonuclease digestion (Figure 9).
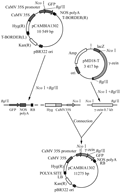 Figure 7 Constructing procedure of the plant expression vector pCB-GFP-γ-zein |
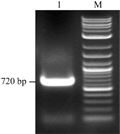 Figure 8 PCR analysis of γ-zeingene fragment |
 Figure 9 Identification of pCB-GFP-γ-zein digested by Ncoâ… and Bglâ…¡ |
The eighth figure exhibited that PCR amplified fragment in size was consistent with the expected size of γ-zein gene (approximately 720 bp); the ninth figure showed that the two fragments were isolated by electrophoresis from recombinant plasmid combining pCAMBIA1302 with γ- zein after double digestion Ncoâ… /Bgl II, of which the molecular weight were approximately 10 kb and 720 bp,respectively, that are consistent with the molecular weight of linear fragments of pCAMBIA1302 and the molecular weight of the γ-zein fragments. The results indicated that the γ-zein fragment was cloned into pCAM- BIA1302 vector, implying that the plant expression vector was well constructed, and the recombinant plasmid was named pCB-GFP-γ-zein.
1.4 Fluorescent detection of onion epidermal cell
DNA of the plasmid pCB-GFP-γ-zein was extracted to be transformed into onion epidermal cells by the method of Biolistic bombardment. Transient expression of the fusion genes were observed after recovering culture with 24~48 hours under the confocal microscope (Figure 10), which preliminary indicated that the genes were normally expressed in onion epidermal cell wall, nucleus and cytoplasm and the expression level from the high was the nucleus, cell wall and cytoplasm in order. Therefore, the plant expression vector pCB-GFP-γ-zein built in this research can be used for future genetic transformation.
|
|
2 Disscussions
GFP as gene transferring reporter and positioning marker has unparalleled superiority in the fields of plant genetically engineering crops, so in recent years it has widely used in the cellular and molecular biology. As the progress of the research, the capacities of the GFP have also been greatly expanded in plant molecular biology which involved in the dynamic monitoring of genetically modified plants, the target protein subcellular localization, the distribution of pathogenic microorganisms and interactions of plants and microbes in the exchanges of material and information and observation among cell organelles.
This study we built pCB-GFP-γ- zein will lay the foundations for further GFP fusion protein expression vector building, γ-zein gene genetic transformation, gene expression and target genes localization in plant cells and biological function analysis.
3 Materials and methods
3.1 materials
The onion (Allium cepa L.) bulb epidermal cells were brought by the market. The plasmid pROK.TG1LK containing gamma-zein gene (Figure 11), plant dual expression vector PCMBIA1302 containing a modified GFP gene (Figure 12) and E. coli DH5α were all provided by Pasture Genetic Breeding Laboratory of the Beijing Institute of Animal and veterinary of Chinese Academy of Agricultural Sciences. Primers were synthesized by Shanghai Invitrogen Company. All of the enzymes and biochemical reagents were purchased from the companies of Shanghai Sangon, Dalian Takara. Gel and PCR, Clean-Up System for recovery and purification of PCR products were produced by Promega Corporation.
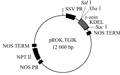 Figure 11 Physic map of the plasmid Prok.TG1K |
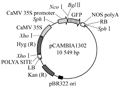 Figure 12 physic map of dual gene expressing vector pCMBIA1302 |
3.2 Methods
3.2.1 Integrity identification of the improved vector pCAMBIA1302
Picking up single colony of Agrobacterium tumefaciens strain LBA4404 containing the plasmid pCAMBIA1302 to inoculate to LB liquid media containing concentrations of 50 mg/L Kan and Str, under the 37°C shaking culture overnight prior to the extraction of plasmid DNA that was digested, respectively, by the restriction enzymes Sphâ… and Xhoâ… . The primers designed for PCR amplification according to the GFP gene sequence as following: 5'-CAGTGGAGAGGGTGAAGGTG-3 ', 5'-CGAAAGGGCAGATTGTGTGG-3'; the amplification procedures followed as : 94°C 5 min in advance, then 94°C 30 s, 47°C 30 s, 72°C 1 min, for 30 cycles; finally 72°C extended for 10 min. the expected amplifying bands should be 538 bp in length.
3.2.2 Construction of plant expression vector
The primers were designed based on the open reading frame (ORF) of γ- zein gene as below 5'-GCATGAGGG TGTTGCTCGTTGCCCT-3' and 5'-ACCATTAGC TCATCTTTCTCACTAGTGTGGGG-3', and then 5' end were introduced into the sequence of two restriction sites Ncoâ… (C leading to CATGG), Bgl II (A leading to GATCT) and protecting the bases (ATAAAAAT and CGCCTAT) Based on the insertion site of target genes in the plant expression vector pCAMBIA1302, the plasmid DNA of pROK.TG1LK as a template for amplifying the full-length γ- zein gene that ligates to the pMD18-T in order to make the intermediate vector that is transformed into competent Escherichia coli DH5α. Picking up positive clones are used for DNA extraction; plasmid DNA is digested by restriction enzyme, and connected to targeted site, of which the plasmid DNA extraction, restriction enzyme digestion, ligation, transformation, competent preparation of E. coli and detection of transformant are followed the conventional methods described in the reference of “Molecular Cloning” (Jin and Li , 1996).
3.3 Biolistic transformation and transient expre- ssion localization
The complete plasmid construct is bombarded into epidermal cells of onion scales by the gene gun (Bio-Rad PDS-1000/He) to identify the function of the plasmid constrct. Stripping onion scales to take the epidermal cells of the inner tender parts, small pieces in size about 4cm X 4cm in were cut with a scalpel to gently peel off the skin cells, and were placed on MS medium under 25°C pre-cultured 4 hours. The plasmid DNA was mixed with 25 μL golded powder (1.6 μmol/L, 60 mg/mL), 25 μL 2.5 mol/L CaCl2, 10 μmol/L, 0.1 mol/L spermidine in the oscillator. Dehydration by 70% ethanol and anhydrous ethanol was applied and then was fully washed twice, finally, suspended in 50 μL of anhydrous ethanol. Taking 10 μL drops on the loading membrane. The vacuum of sample chamber was set under 25 Pa and 1300 psi; the pre-cultured onion epidermal cells were placed on the platform apart from 25 cm in distance of the loading film for bombardment; and the bombarded onion epidermal cells were cultured at dark moist environment under 28°C, for 24~48 hours.
Confocal microscope (Model: Nikon TE2000E) was emplyed to detect transient expression of green fluorescence in onion epidermal cells with the blue excitation light (488 nm) and 10×eyepiece.
Author contributions
YZ mainly engaged in designing the program, doing the specific test and wrote paper; SQB engaged in the program and revised their paper; CL guided the specific experimental methods; DXL and YCD revised the paper; YX W took part in the DNA isolation and Southern blot experiments. All authors have read and approved the final manuscript.
Acknowledgements
This work was funded by National Science and Technology Support Project (2011BAD17B03). We thanked the collaboration of Forage Breeding Laboratory of Institute of Animal Science, Chinese Academy of Agricultural Science.
References
Chiu W.L., Niwa Y., and Zheng W., 1996, Engineered GFP as vital reporter in plants, Curr. Biol., 6: 325-330
http://dx.doi.org/10.1016/S0960-9822(02)00483-9
Coleman C.E., Larkins B.A., 1999, The prolamins of maize, In:Shewry P.R., and CaseyR., eds (Dordrecht, 1998, The Netherlands: KluwerAcademic Publishers), pp.109-139
Elliott A.R., Campbell J.A., Dugdale B., Brettell R.I.S., and Grof C.P.L., 1999, Green-fluoreseent protein faeilitates rapid in vivo deteetion of genetieally transformed plant cells, Plant Cell Rep., 18: 707-714
http://dx.doi.org/10.1007/s002990050647
Esen A., 1987, Proposed nomenclature for the alcohol-soluble proteins (zeins) of maize (Zea mays L.), J. Cereal Sci., 5: 117-128
http://dx.doi.org/10.1016/S0733-5210(87)80015-2
Huang G.C., Zhu S.W., Dong Y.M., and Sun J.S., 1998, Green fluorescent protein and its application in plant research, Chinese bulletin of botany, 15(5): 24-30
Jacques L., 2002, A linear model for quantitating the accumulation of zeins and their fractions (α+δ, β&γ) in developing endosperm of wild-type and mutant maizes, Plant Science, 163(1): 111-115
Jin D.Y., Li M.F., trans., 1996, Molecular cloning experiment manual, 2nd ed, Beijing, Science Publishing Press, pp.42-68
Shewry P.R., and Casey R., eds., 1999, Seed Protein, Kluwer Academic Publishers, Netherlands, pp.109-139
Shimomura O., Johnson F.H., and Saiga Y., 1962, Extraction, purification and properties of aequoria, a bioluminescent protein from the luminous hydromeddusan, Aequorea, Cell Comp Physiol, 59: 223-229
http://dx.doi.org/10.1002/jcp.1030590302
PMid:13911999
Sumilkumar G., Rathore K.S., 2001, Transgenic cotton; factors influencing Agrobacterium-mediated transformation and regeneration, Mol. Breed., 8: 37-52
http://dx.doi.org/10.1023/A:1011906701925
Wang H.Y., Zhou S.B., Chang Z.Z., Ma Y., and Qin W.H., 2004, Researching progress of green fluorescent protein, Biotechnology, 14: 70-72
Wilson C.M., 1991, Multiple zeins from maize endosperm characterized by reverse-phase high performance liquid chromatography, Plant Physiol., 95: 777-786
http://dx.doi.org/10.1104/pp.95.3.777
PMid:16668053 PMCid:1077605
Wooa Y.M., Hub D.W.N., Larkins B.A., and Jungb R., 2001, Genomics analysis of genes expressed in maize endosperm identifies novel storage proteins and clarifies patterns of zein gene expression, Plant Cell, 13: 2297-2317
PMid:11595803 PMCid:139160
Xie S.Y., 2003, Researching progress of rumen protection sulfur amino acid, Siliao Guangjiao (Feed China), 11: 21-24
Zhao P.O., Xie K., and Guo Z.J., 2006, Transformation of tobacco plants by rice OsWRKY10-GFP fusion gene and observation of subcellular localization, Zhejiang Nongye Xuebao (Acta Agriculturae Zhejiangensis), 18 (3): 159-162
. PDF(702KB)
. FPDF(win)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Yu Zhang
. Shiqie Bai
. Cong Li
. Daxu Li
. Yongchang Deng
. Yongxin Wang
Related articles
. Maize
. γ-zein gene
. Green fluorescent protein (GFP)
. Plant expression vector
. Subcellular localization
Tools
. Email to a friend
. Post a comment